Francisellosis: clinical aspects of the disease and control strategies
Source: O Presente Rural, Edicao 205, November 2021, www.opresenterural.com.br (originally published in Portuguese)
Francisellosis: clinical aspects of the disease and control strategies
Santiago Benites de Padua, DVM, MSc in aquaculture – Aquaculture Product Manager – Vaxxinova Brazil
Francisellosis, also known as bacterial visceral granulomatosis, is a disease caused by a facultative intracellular Gram-negative bacteria called Francisella orientalis. This agent has established itself as an important pathogen in tilapia farming throughout Brazil, emerging at the beginning of the last decade in Brazilian tilapia culture. Since then, francisellosis has been gaining ground and causing sanitary problems in all farming systems, such as net cages and earthen ponds, as well as HDPE circular aquaculture tanks for outdoor fish farming system.
Currently, the disease is endemic in the main areas of tilapia farming in Brazil, causing seasonal disease in the São Francisco river basin, occurring in several States of Northeastern Brazil, as well as in South-Central Brazil, where it is particularly able to cause greater losses since weather conditions favour the persistence of the disease for a large part of the year.
Risk factors
A drop in water temperature is the main risk factor for the occurrence of francisellosis on farms. Thus, the disease gains greater importance in late autumn, winter, and early spring, characterizing itself as a seasonal disease. Generally, francisellosis starts with a water temperature below 25 °C, but in some regions of Brazil we have observed severe cases with temperatures ranging from 25 to 27 °C at specific times of the year, especially during early spring.

Santiago Benites de Padua, DVM, MSc in aquaculture – Aquaculture Product Manager – Vaxxinova Brazil
Other risk factors can act on the increase of susceptibility of fish to this pathogen’s challenge, with particular attention to stocking density, in addition to the physicochemical parameters of the water, especially the alkalinity of earthen ponds. Empirically speaking, we observed that during periods of high risk for the occurrence of francisellosis, earthen ponds with low alkalinity apparently present the disease more aggressively, accumulating greater losses related to the disease.
Risk Group
Traditionally, francisellosis was a disease that affected young forms of tilapia as the main risk group for the disease, particularly affecting the farming stages of fry and juveniles. However, over the years, we have observed that this bacterium has increasingly infected larger fish, which can cause significant mortality outbreaks in animals between 200-400 g live weight, with the costs of treatment using antibiotics have a great impact on tilapia production cost. Especially in the season of the temperature drop in 2021, we observed that the weight of fish diagnosed with francisellosis increased dramatically compared to recent years (Figure 1), which possibly indicates the adaptability of this bacteria in animals in the finishing stage of the farming cycle.
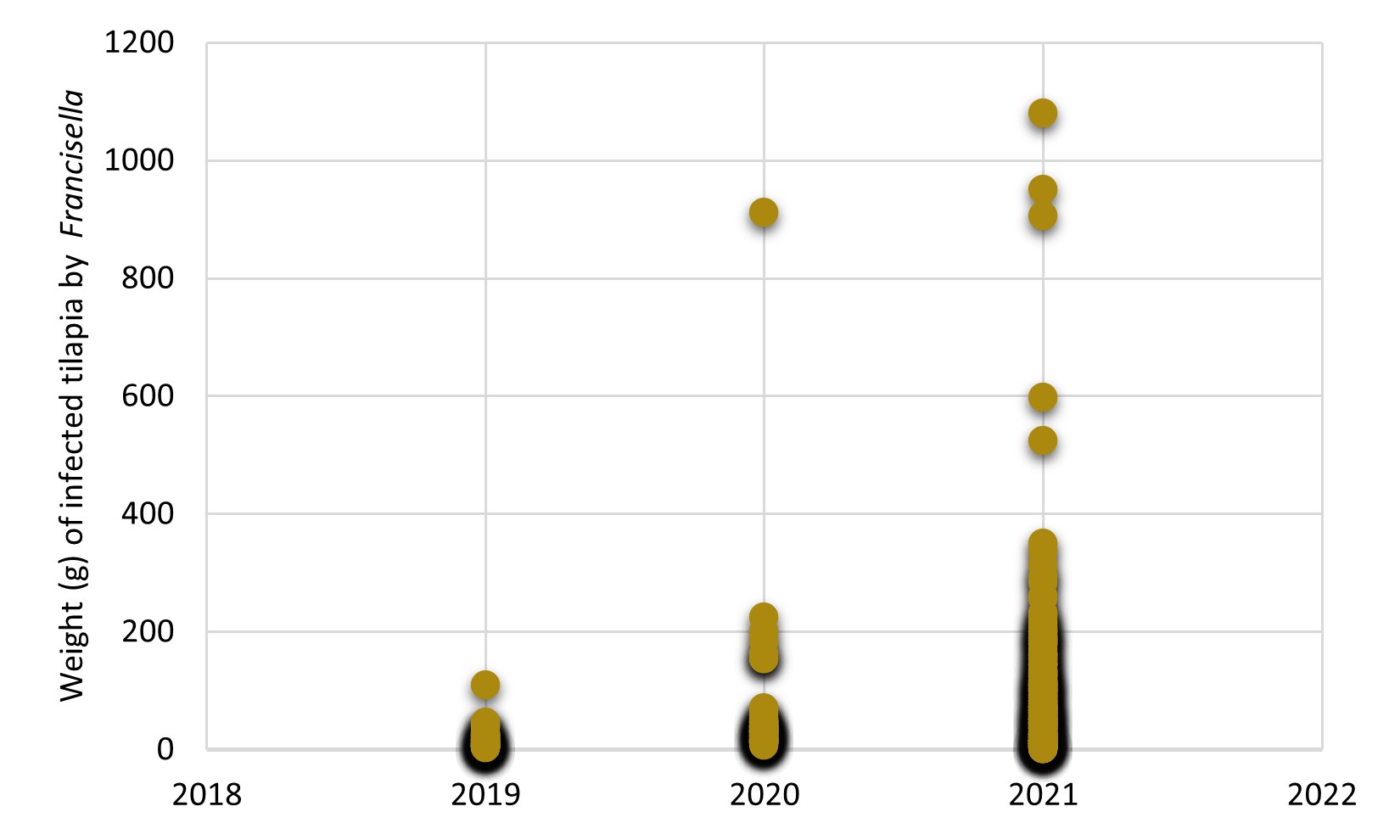
Figure 1. Weight (g) of tilapia diagnosed with Francisella orientalis between 2019 and 2021. Data recorded in the diagnostic history of the AQUAVET Laboratory/UFMG, under the responsibility of Prof. Dr Henrique Figueiredo.
Pathogenesis
Francisella orientalis is a facultative intracellular bacterium, which can live inside the defence cells that make up the immune system of animals, especially inside macrophages, being the main cell line responsible for phagocytosis and elimination of several pathogens. After infecting macrophages, Francisella uses several strategies to evade tilapia’s defence mechanisms. Adding to this process, the infection induces an intense inflammatory reaction mediated by the migration of macrophages to target organs, with the spleen and kidneys being the most affected by the disease, starting the formation of multiple granulomas in these organs that can be observed with the naked eye in advanced stages of the disease (Figure 2).
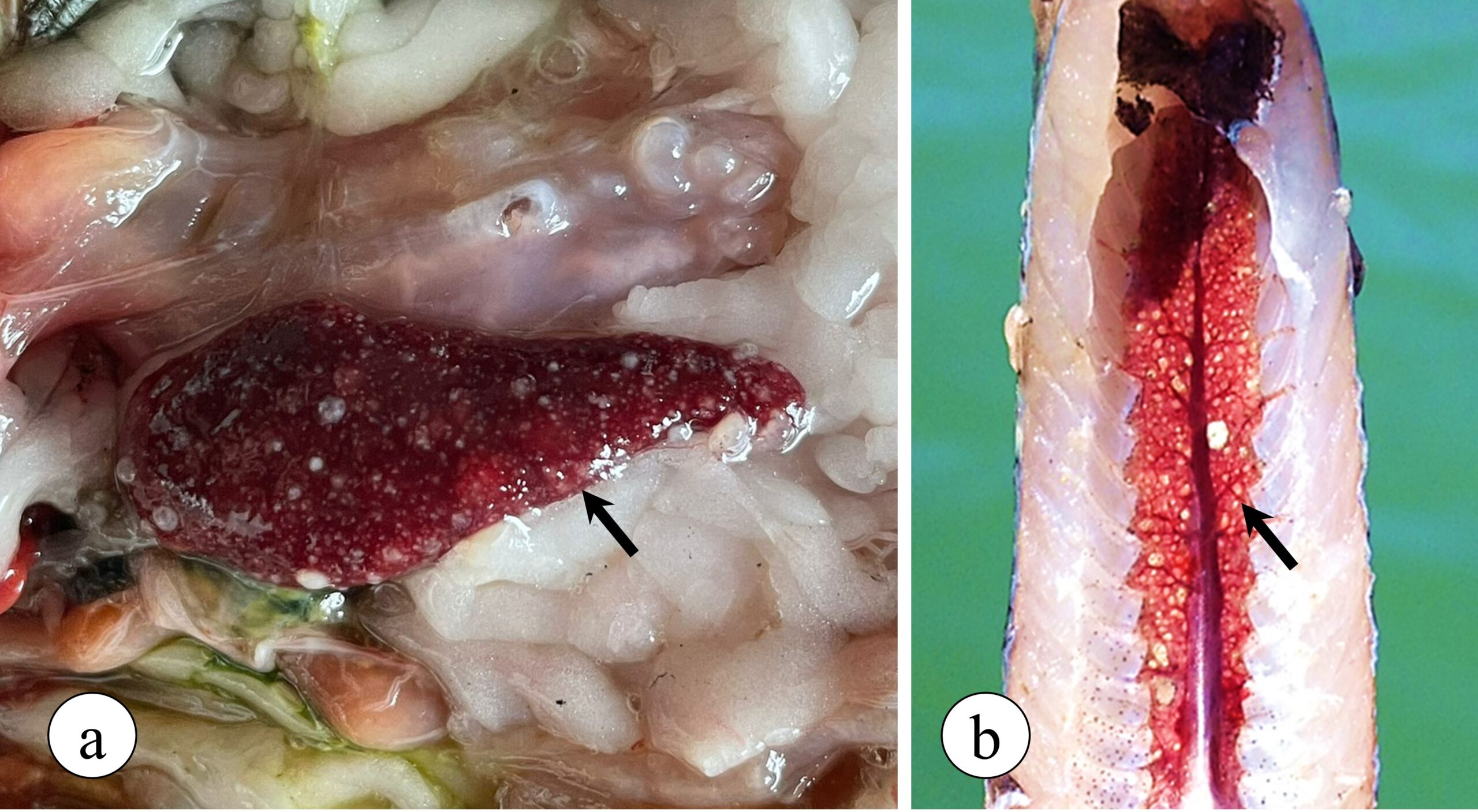
Figure 2. Granulomatous lesions observed in Nile tilapia with francisellosis. (a) Spleen exhibiting multiple granulomas (arrow) in addition to enlargement of the organ (splenomegaly). (b) Caudal kidney showing multiple granulomas.
Interestingly, granulomatous diseases are usually chronic pathological processes, with a long course of disease in most animals, which may involve months of clinical evolution. However, in the case of granulomatous diseases in tilapia this reality is somewhat different since, after the exposure of susceptible animals to the bacteria, granulomas can be microscopically observed as early as 7 days after infection. Likewise, the onset of clinical signs and the occurrence of mortality associated with this disease, as well as the observation of granulomas with the naked eye, start between 14 and 21 days after infection in a field situation.
With the progression of the disease in the infected animal, especially associated with the maintenance of the disease’s risk factors, francisellosis can progress and cause visceral granulomatosis in several other organs, such as the gills (Figure 3a), liver (Figure 3b), intestines, heart, brain and eyes, as well as bacterial granulomatous myositis. This latter, in turn, is an infection in the fish musculature, causing numerous small black spots, measuring around 1 mm, in the fillet of infected animals. These black lesions in the fillet are related to the presence of granulomas containing melanomacrophages with melanin pigment around the inflammatory focus and are characterized as one of the causes for fillet disposal in slaughterhouses.
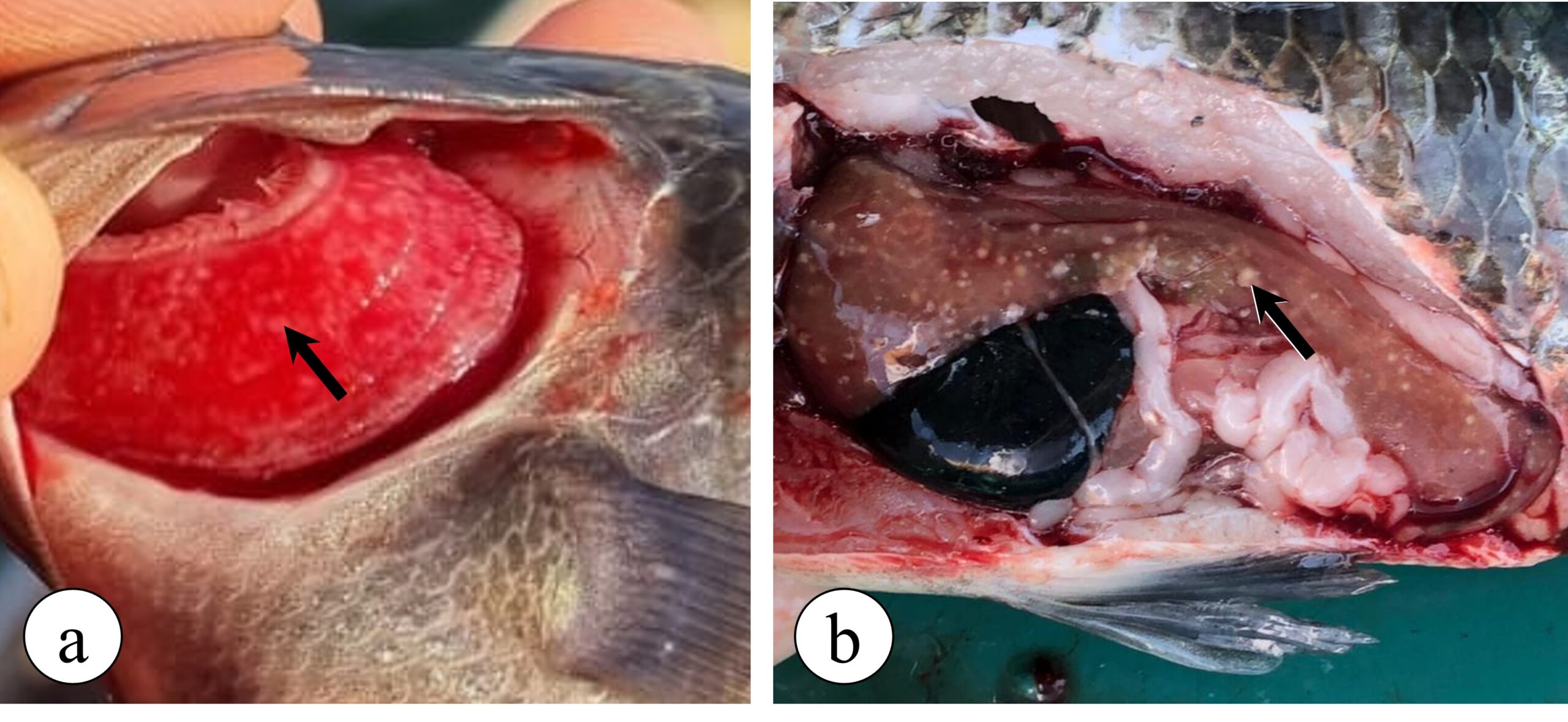
Figure 3. Granulomatous lesions observed in Nile tilapia with francisellosis. (a) Gill granulomatosis (arrow), where numerous white spots (granulomas) can be seen on the gill filaments. (b) Hepatic granulomatosis (arrow), where several white spots can be seen in the liver of a diseased tilapia fish, indicating the presence of granulomas.
Impact on animal performance
When francisellosis enters in a fish farm, one of the main notable impacts is a drop in zootechnical performance. Traditionally, when the period of water temperature dropping starts, tilapia naturally reduce their appetite, as well as reduce their growth rate and worsen their feed conversion. However, this condition tends to worsen dramatically with the occurrence of francisellosis during this period, in which animals can completely stop eating and zootechnical indexes drastically worsen. This way, directly impacting production costs, increasing the farming cycle, in addition to making it difficult for the proper use of medicated feed due to the impact on fish appetite.
The drop in zootechnical performance in fish may go unnoticed by some farmers, as fish naturally slow down their growth during periods of ‘drops in water temperatures’. However, for more experienced tilapia farmers, the delay in the animals’ growth and the worsening of the feed conversion rate is noticeable with the simultaneous occurrence of cold and Francisella orientalis infection.
Diagnosis of francisellosis
The presumptive clinical diagnosis of francisellosis is easily performed in the field, based on the observation of the main clinical signs, such as a sharp decrease in appetite during winter periods, associated with reduced animal growth and, mainly, by the observation of granulomas in the organs of moribund fish, especially in the risk group (fry and juveniles). However, the confirmation of the clinical suspicion observed in the field is carried out through laboratory tests, using specific techniques for the isolation and identification of Francisella orientalis. Or even through the combination of molecular tools for the detection of bacterial DNA, such as the PCR reaction, these being the main methods used for the definitive diagnosis of the disease.
On the other hand, in recent years another disease has emerged in Brazil, Edwardsiellosis, which is caused by the Gram-negative bacteria Edwardsiella tarda and has been easily confused with francisellosis. This disease can also cause granulomatous disease in young forms of tilapia, and it is not possible to differentiate the two diseases simply based on the clinical history and clinical manifestations in the field. Therefore, obtaining a definitive diagnosis through a complete laboratory analysis becomes essential for the correct identification of the cause of granulomatous disease in tilapia.
Disease treatment
Once the disease has established itself on the breeding farm, its occurrence will be annual and difficult to eradicate. Therefore, farmers need to establish a routine of frequent monitoring of the occurrence of francisellosis, based on clinical analyses with the observation of granulomas in animals from the risk group during periods of drops in water temperature, along with the confirmation of the diagnosis in the laboratory. Following that, the most effective way to control the outbreak in a tilapia flock is by using feed medicated with antibiotics, with early diagnosis being the key to success in achieving a good response in treating diseased fish. However, scientific data concerned to antibiotics use in francisellosis control in Nile tilapia are lacking. As well as data on efficacy, safety for fish and residues in the fillet during drop in water temperature when applying antibiotics. So it becomes a challenge to control disease occurrence. Therefore, the implementation of a well-designed vaccination program based with tailor-made vaccines are the best way to control this threat in tilapia farm.
Vaccination
Currently, the best strategy for preventing infectious diseases in any animal production activity, as well as for human health, is based on the use of vaccines. In Brazil, licensed vaccines against francisellosis are not yet available. However, tailor-made vaccines, also known as autogenous vaccines or custom made vaccines, have gained an important place in this market, being the best alternative for disease control by tilapia farmers.
To produce an autogenous vaccine against francisellosis, field technicians and/or farmers need to contact an animal health company licensed by MAPA (Brazilian Ministry of Agriculture, Livestock and Supply) for the production and sale of autogenous vaccines. From this stage onwards, a field veterinarian will collect sick fish on the target farm, where clinical cases of the disease are occurring, and send the clinical samples to a specialized laboratory in fish diagnosis. After obtaining the Francisella orientalis strain from sick fish, a prescription for the use of an autogenous vaccine against this pathogen is made and the authorization for the production of the vaccine is requested to MAPA.
After obtaining the approval by the Ministry for the production of an autogenous vaccine, which occurs within 48 hours after the request is made, the licensed veterinary vaccine company receives the field isolate and prepares the production of “seeds” for producing the vaccine using the isolated bacteria of sick fish (Figure 4). This way, we provide the high antigenic specificity needed to control the disease, using bacterial strains occurring in the field, obtaining maximum protection for the vaccinated flock with a tailor-made product. In addition, another great benefit of autogenous vaccines is the agility in delivering solutions against emerging diseases, such as francisellosis, which still do not have licensed vaccines on the global market, providing an effective solution to reduce the use of antibiotics to treat the disease, in addition to promoting better animal health and welfare during the pathogen-protected production cycle.
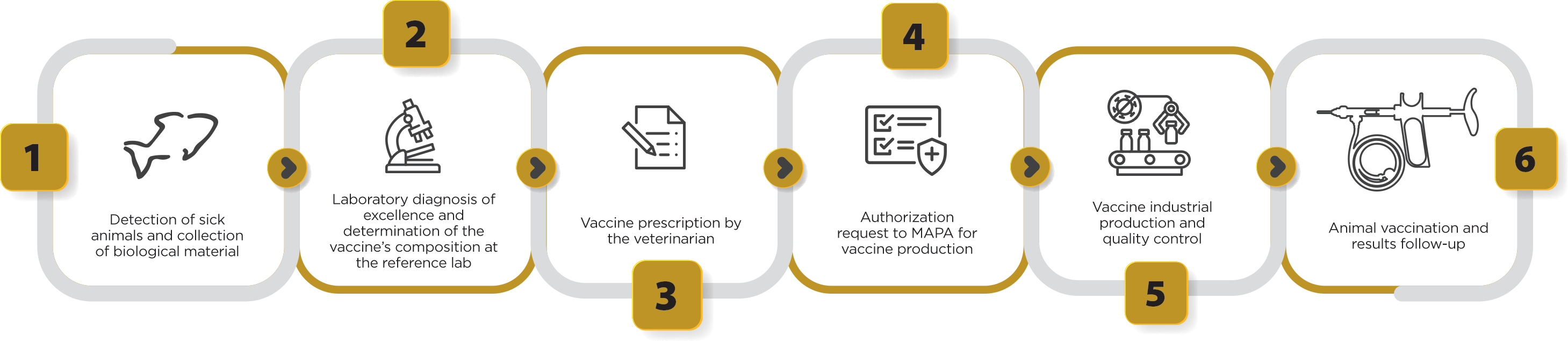
Figure 4. Stages of clinical sample collection, laboratory diagnosis, vaccine prescription by the veterinarian, authorization request to MAPA and production of autogenous vaccines used for tilapia culture.
Disclaimer: this article is a translation from the publication in O Presente Rural in Portuguese. The content relates to the use of the products and its field results in Brazil and Latin American countries.
A reference to any product/service in this article does not imply that such product(s) with the same specifications, is or will be available at your location. Any product shown or referred to on this website may be subject to different regulatory requirements depending on the country of use.
Please contact your local Vaxxinova representative for more information.
