Autogenous vaccines: customizing health management in tilapia culture
Source: Panorama da AQÜICULTURA | Vol. 31, Edição 185 | panoramadaaquicultura.com.br (originally published in Portuguese)
Autogenous vaccines: customizing health management in tilapia culture
Santiago Benites de Pádua, DVM, MSc in Aquaculture – Aquaculture Marketing Manager – Vaxxinova Brazil
Nile tilapia farming has experienced vigorous growth cycles in recent years and is currently the main fish species farmed in Brazil, surpassing the sum of all other fish species that have dominated the production scene for several years. However, coupled with the increase in the production of any animal species in intensive farming, emerging pathogens can represent a significant threat to activity growth. This reality, unfortunately, is no different when it comes to Brazilian tilapia culture, where several infectious agents have emerged in the last decade. Such are Francisella orientalis, Streptococcus agalactiae serotype III, Streptococcus dysgalactiae, Lactococcus garvieae, Edwardsiella tarda and iridovirus ISKNV (Infectious Spleen and Kidney Necrosis Virus).
For tilapia farmers, the health management of diseases that affect farming is, without a doubt, one of the key factors to obtain success and profitability in this activity. However, disease management becomes even more challenging when new pathogens and genetic variants of pathogenic agents already established in farming centres often emerge. These new agents do not have available solutions based on licensed vaccines, which take years to be developed, approved and launched on the market. In this scenario, autogenous vaccines, also referred to as custom made vaccines, gain an important space as the most agile tool to deliver effective solutions for tilapia farmers against emerging diseases in their activity.


Stages of autogenous vaccine production
Diagnosis
The first step towards the implementation of an active immunoprophylaxis program based on the use of autogenous vaccines is the diagnosis of the main causes of mortality in farmed fish. To that end, clinical samples must be properly collected by healthcare professionals, ensuring the correct storage and transportation to the diagnostic laboratory. The number of collected animals, as well as the frequency of diagnosis to be performed, are key points for the success in developing a vaccination program based on autogenous vaccines, taking into consideration the seasonality of the diseases that occur on the breeding farm. This definition is essential since the use of multivalent vaccines, which have multiple types of pathogens in their formulation, does not always deliver the best protection results.
No less important than the proper sampling of biological material, it is essential for the laboratory that will receive that material to have the appropriate expertise and technology for the accurate identification of the infectious agents obtained, as well as different typing techniques and tools for proper selection of the agents that will compose the vaccine formulation. Based on these assessments, combined with the epidemiological situation in the field, the healthcare professional will prescribe the best combination of agents in a vaccination program using autogenous vaccines.
Authorization request to MAPA for vaccine production
With a diagnosis in hand and a veterinary prescription for the use of the vaccine, the veterinary industry qualified to produce biological products will request authorization from MAPA to produce autogenous vaccines. The diagnostic reports and indication of agents that will compose the vaccine will be sent, including details on target farms, adjacent farms and/or integration systems, with an indication of the volume of doses to be produced. At this stage, the response is obtained within 48 hours and the procedure is then authorized by the regulatory body, provided that the requirements previously established in normative instructions are fulfilled.

Industrial production and in vitro quality control
After receiving the authorization from MAPA to produce the autogenous vaccine, the microorganisms isolated by the diagnostic laboratory are transferred to the facilities qualified to produce the vaccine, where the seed bank for the production of batches of autogenous vaccines will be created and the first quality control analyses will be carried out on the seeds, including the evaluation of the viability and purity of the produced stock. After this stage, the industrial production of bacterial suspensions starts, using controlled conditions of microbial culture, until obtaining high concentrations of bacteria necessary for the inactivation and formulation of vaccines. All stages of microbiological cultivation and inactivation of bacterial suspensions undergo analysis and quality control of the intermediate product, in order to ensure that there has been no contamination of the culture, as well as that there has been proper growth (bacterial titer). Finally, the inactivation test is performed, in which it is evaluated whether there was an adequate inactivation of the bacteria present in the suspension by the action of the inactivating agent.

Santiago Benites de Pádua, DVM, MSc in Aquaculture Aquaculture Marketing Manager – Vaxxinova Brazil
After its release by the quality control team, the inactivated bacterial suspension is renamed as antigen. From then on, the finished product is formulated, in which different oils and pro-inflammatory factors are combined, together with stabilizing products and adequate amounts of antigens, mixed in a procedure called oil emulsion. This technique allows mixing oil-based adjuvants with different antigens and water-based pro-inflammatory agent. The purpose is to promote the deposition of the vaccine in the fish’s organism for a longer period, achieving a strong production of antibodies, as well as a long duration of protection of the vaccinated animals. Finally, after packaging the vaccine, the last in vitro analyses are carried out, including the stability of the emulsion, sterility of the finished product, in addition to other physical-chemical tests that make up the laboratory’s quality control protocol.
In vivo quality control
The last industrial stage of autogenous vaccine production is the in vivo quality control of the finished product, including a series of analyses carried out before releasing the autogenous vaccines for sale. This stage involves using the formulated and packaged vaccines that have already been previously approved by in vitro analyses. Safety tests, also called innocuity tests, are performed, in addition to the efficacy test, using an animal model to check these analyses.
At Vaxxinova Brazil, the animal model used is the target species itself with the indication for the use of autogenous vaccines, using Nile tilapia juveniles raised under laboratory conditions (Figure 1). It is important to point out that all analyses are carried out in animal facilities duly registered with regulatory bodies, such as CONCEA (National Council for the Control of Animal Experimentation), following all international standards of good practice in clinical studies, ensuring animal welfare.

Figure 1. Facility designed for development and in vivo quality control procedure for autogenous vaccines at Vaxxinova Brazil.
Safety tests
To carry out the safety test, a standard number of animals is subjected to vaccination using twice the conventional dose indicated in the product package insert, to which 0.1 mL of vaccine is administered per fish. These animals are monitored for 21 days after vaccination for the occurrence of adverse effects, such as behavioural changes, changes in fish colour, appetite, among other clinical aspects. At the end of the evaluation period, the animals are euthanized and necropsied to evaluate the visceral changes related to the vaccine and then the Spielberg score is determined, through which the inflammatory reactions induced by the vaccine can be evaluated.
Efficacy test
Unlike in the safety test, in the efficacy test the micro dose of the vaccine is used, with the same dose indicated in the product label insert for use in field-raised fish, which corresponds to 0.05 mL/fish. After vaccination, the fish are kept under laboratory conditions, in an environment where water quality conditions are controlled, including temperature, oxygenation, as well as photoperiod control. After 21 days post-vaccination, during which the vaccinated fish are completely protected, these animals are submitted to a bacterial challenge, through which known doses of bacteria that make up the vaccine formulation are inoculated. Finally, the animals are monitored for another 15 days post-challenge, where the percentage of vaccine protection can be calculated by calculating the RPS (Relative Percent Survival).
Through these two in vivo quality control analyses, we are able to ensure that the autogenous vaccines are safe to be administered to the flock of field-raised tilapia, as well as deliver the necessary efficacy for adequate protection of the animals by the agents that make up the vaccine. This way, we are able to provide high reliability in terms of product safety and guarantee vaccine responses induced by the autogenous vaccines of the Govaxx® range.
Figure 2 shows all the stages of autogenous vaccine production. In general, it takes around 3 months to produce a batch of Govaxx® autogenous vaccines, from the first field services, as well as the in vitro and in vivo quality control period, until the product is released for sale. However, this period may vary according to the infectious agent that will be added to the vaccine formulation, as well as according to the number of diagnostic analyses necessary for the selection of agents that will compose a vaccination program.
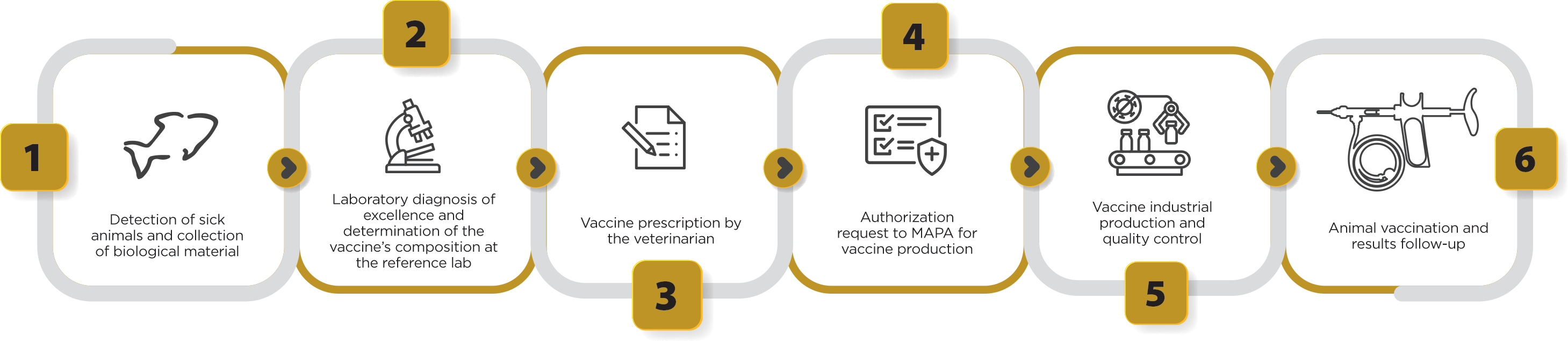
Figure 2. Stages of clinical sample collection, laboratory diagnosis, vaccine prescription by the veterinarian, authorization request to MAPA and production of autogenous vaccines used for tilapia culture.
Advantages of autogenous vaccines
The greatest advantage of autogenous vaccines lies in the fact that they constitute the main tool of health control that can be used in conditions of emergence of new pathogens. As previously mentioned, the average time to obtain an autogenous vaccine batch can take around 3 months. On the other hand, for licensed vaccines, it takes years before a commercial vaccine is made available to tilapia farmers, which could represent significant losses to the sector.
In addition to the relatively short time related to the product delivery, another great benefit of autogenous vaccines is the high antigenic specificity of their formulation. Since genetic variants and bacterial serotypes that occur on the breeding farms are used to compose the product formulation. Those agents are monitored within an epidemiological surveillance program linked to the
diagnostic services of the Govaxx® autogenous vaccine program. This way, we provide an effective solution against vaccine failures that can occur when the licensed vaccine does not provide adequate protection against the pathogen variants that occur in the field.
With the implementation of a vaccination program based on autogenous vaccines, we were also able to provide a significant reduction in the use of antibiotics, promoting greater food safety for tilapia consumers, as well as superior animal welfare, coupled with the reduction of production costs, in addition to actively contributing to reducing the risk of emergence of multi-antibiotic resistant bacteria. Therefore, in an increasingly competitive and demanding market, where tilapia farmers increasingly seek to adapt their production to higher quality levels to access different markets, the best strategy for the health management of their production is based on the services rendered in a vaccination program with Govaxx® autogenous vaccines.

Disclaimer: this article is a translation from the publication in Panorama da Aquicultura in Portuguese. The content relates to the use of the products and its field results in Brazil and Latin American countries.
A reference to any product/service in this article does not imply that such product(s) with the same specifications, is or will be available at your location. Any product shown or referred to on this website may be subject to different regulatory requirements depending on the country of use.
Please contact your local Vaxxinova representative for more information.
